Driving Towards a New Approach to Idiopathic Pulmonary Fibrosis (IPF)

What is a Clinical Trial?
A clinical trial, also known as a research study, helps researchers answer questions about certain conditions and diseases and their treatments. By choosing to participate in clinical trials, participants help the research team answer questions about how the drug works and monitor to see if it is safe to use before it is released into the market. This is important to help discover new treatments for a disease and aid in detecting, diagnosing and preventing the disease from becoming worse. Clinical trials assist doctors in understanding a potential treatment’s safety and how effective the treatment is, what the potential side effects may be and what works or doesn’t work before they begin prescribing them to more people. If clinical trials are not conducted, doctors will not be able to determine if new treatments actually work.
The information provided on this webpage will give you details about the GRI-0621-IPF-02 clinical trial for Idiopathic Pulmonary Fibrosis (IPF) and help you decide if you want to become involved.

Learn About a New Clinical Trial for Participants With IPF
GRI Bio Inc. is a clinical-stage biopharmaceutical company focused on fundamentally changing the way inflammatory, fibrotic and autoimmune diseases are treated. GRI Bio is currently running a Phase 2a clinical trial called GRI-0621-IPF-02 for participants with Idiopathic Pulmonary Fibrosis (IPF).
The GRI-0621-IPF-02 clinical trial aims to determine whether GRI-0621 is safe and tolerable for the treatment of IPF. In this 18 week study, you will have regular check-ups, frequent blood tests and receive more medical supervision than you would under normal treatment procedures.
The information obtained from this study may help treat future individuals with IPF and will provide important information about how well people respond to GRI-0621. The study treatment you will receive may or may not improve your disease condition.
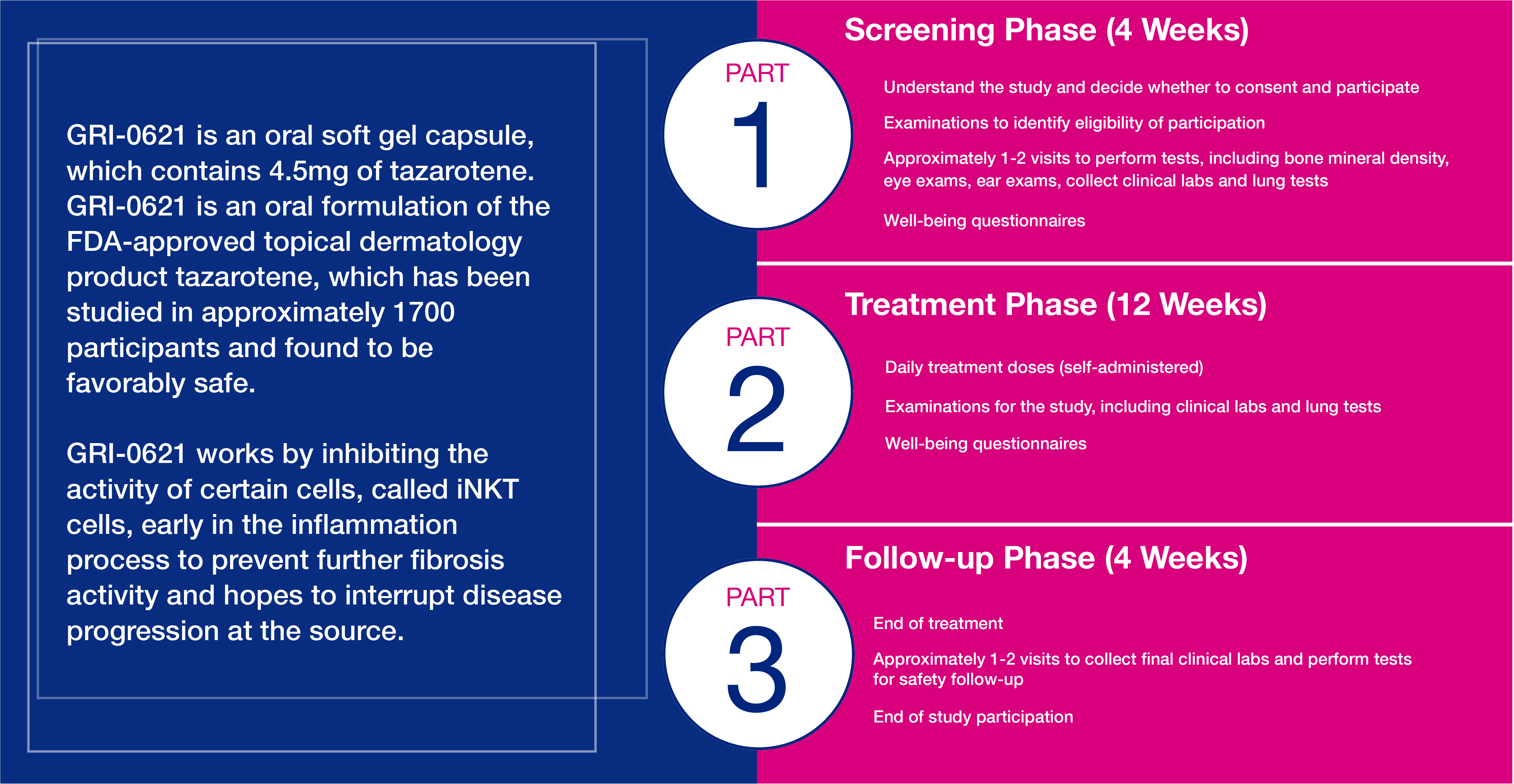
What is GRI-0621
What to Expect
You will have regular tests and assessments performed during the study, which include:
Clinical Sites
US Sites:
FLA Pulmonary, Allergy, and Sleep Medicine
4500 San Pablo Rd. S
Jacksonville, FL 32224
Principal Investigator:
Dr. Augustine Lee
lee.augustine@mayo.edu
Primary Contact:
Jhozia Duncan
Duncan.Jhozia@mayo.edu
Medical University of South Carolina
96 Jonathan Lucas Street
Charleston, SC 29425
Principal Investigator:
Dr. Aravind Menon
Primary Contact:
Asha Simmons, Nadine O’Connor
simmasha@musc.edu
Montefiore Medical Center
111 East 210th Street 5th Floor
Bronx, NY 10467
Principal Investigator:
Dr. Ali Mansour
amansour@montefiore.org
Primary Contact:
mesuarez@montefiore.org
lcastellan@montefiore.org
Newport Native MD
1501 Superior Ave, Suite 202
Newport Beach, CA 92663
Principal Investigator:
Dr. Ryan Klein
Primary Contact:
Brandon Vu
Brandon@newportnativemd.com
Southeastern Research Center
29332 Lyndhurst Ave, Suite 110
Winston Salem, NC 27103
Principal Investigator:
Dr. Benjamin Bregman
Primary Contact:
Lauren Miller
lmiller@southeasternresearchcenter.com
UK Sites:
Norfolk & Norwich University Hospital
Room UG_06, Rosalind Franklin Road
Norwich, Norfolk, England, NR4 7uQ
Principal Investigator:
Prof. Andrew Wilson
a.m.wilson@uea.ac.uk
Primary Contact:
Lisa Hudig
Bethany Bridgwood
bethany.bridgwood@nnuh.nhs.uk
Rebecca Angove
rebecca.angove@nnuh.nhs.uk
Oxford University Hospitals NHS Foundation Trust
EMCRF C/- CCVTM Reception
Churchill Drive Headington
Oxford OX3 7LE
Principal Investigator:
Peter Saunders
Peter.saunders@ouh.nhs.uk
Primary Contact:
Kafayah Babatunde
abodunrin.babatunde@ouh.nhs.uk
Royal Devon University Healthcare NHS Foundation Trust
Respiratory Research Team
Room F8, Bowmoor House
Barrack Road
Exeter EX2 5DW
Principal Investigator:
Dr. Michael Gibbons
michael.gibbons2@nhs.net
Primary Contact:
Ana-maria Adam
ana-maria.adam@nhs.net
Royal Infirmary of Edinburgh
51 Little France Crescent
NHS Lothian
Room S1634
EH164SA Edinburgh GB
Principal Investigator:
Dr. Nikhil Hirani
Primary Contact:
Sibusiso Dhladhla
sibusiso.dhladla@ndorms.ox.ac.uk
Sarah McNamara
sarah.mcnamara@nhs.scot
Royal Papworth Hospital
Heart and Lung Research Institute
Royal Papworth Hospital
Biomedical Campus, Papworth Road, Trumpington Cambridge,
Cambridgeshire, UK CB2 0BB”
Principal Investigator:
Dr. Helen Parfrey
helen.parfrey@nhs.net
Primary Contact:
Jenny Castedo
jenny.castedo1@nhs.net
University College London Hospitals
UCLH Clinical Research Facility
170 Tottenham Court Road
London W1T 7HA
Principal Investigator:
Dr. Jo Porter
joanna.porter@ucl.ac.uk
Primary Contact:
Donna Basire
d.basire@ucl.ac.uk
University Hospital Birmingham, Queen Elizabeth Hospital
Mindelsohn Way
Edgbaston, Birmingham
B15 2GW
Birmingham, West Midlands, UK
Principal Investigator:
Dr. Anjali Crawshaw
anjali.crawshaw@uhb.nhs.uk
Primary Contact:
Emma Bruce
emma.bruce@uhb.nhs.uk
Western Health and Social Care Trust (WHSCT)
C-TRIC Altnagelvin Hospital
Glenshane Road
Derry/Londonderry
BT47 6SB
Principal Investigator:
Nazia Chaudhuri
Nazia.chaudhuri@nhs.net
Primary Contact:
Ryan Campbell
ryan.campbell@westerntrust.hscni.net
Valerie Mortland
valerie.mortland@westerntrust.hscni.net
Australia Sites:
Concord General Repatriation Hospital
Hospital Road,
Concord, NSW, 2139
Principal Investigator:
Dr. Elizabeth Veitch
Primary Contact:
Mayrose Chan
Mayrose.Chan@health.nsw.gov.au
St. George Hospital
Gray Street,
Kogarah, NSW 2217
Principal Investigator:
Ajantha Raguparan
Primary Contact:
Mitchell Taylor
Mitchell.Taylor@health.nsw.gov.au
Frequently Asked Questions:
You have the right to ask questions about the GRI-0621-IPF-02 clinical study and to have those questions answered to your satisfaction.
What is the purpose of this study?
This study aims to determine whether GRI-0621 is safe and tolerable for the treatment of IPF.
How long is the study?
If eligible to participate, you will be in the study for approximately 18 weeks, which includes approximately 12 clinic visits.
Will I receive study drug?
If you are eligible to participate in this study, you may receive GRI-0621 (active study drug) or placebo. A placebo is a “dummy treatment” that looks like the study drug but has no active ingredients.
How is the study drug dosed?
Both GRI-0621 or placebo will be provided as 4.5mg of a soft gel capsule, which will be taken orally (by the mouth) once a day for 12 weeks.
There is no dosing diary required to complete.
Do I need to stop my current IPF medication?
No, if you are already on approved IPF therapies, you will remain on your current medication for the entirety of the study.
Is there a cost to participate?
No, you will receive study drug at no charge and there will be no charge to you for any procedures done in this study. You will also be
reimbursed for costs related to participating in the study.
Will I receive any compensation?
Your participation in the study may be compensated. Please speak with your doctor for further information regarding participation compensation.
What if I change my mind?
Your participation in the study is completely voluntary. If, at any point, you decide that you no longer want to proceed, you can opt-out – it will not impact the care you are currently receiving for your IPF.

