GRI-0621
GRI-0621
In development for the treatment of idiopathic pulmonary fibrosis (IPF).
GRI-0621 is a small molecule RAR-βɣ dual agonist that inhibits the activity of human iNKT cells. GRI-0621 has been shown to reduce aminotransferases and other LFTs in patients and improve fibrosis in multiple disease models. GRI is repurposing GRI-0621 as a once-daily oral capsule for the treatment of IPF with the potential to expand into additional fibrotic indications.
Key Highlights:
-
Targets upstream in the inflammatory cascade providing potential for greater efficacy
-
Favorable safety profile demonstrated in prior late stage studies
-
iNKT inhibition demonstrated fibrosis resolution in multiple animal models
-
Extensive IP protection with issued medical use patents and market LOE through 2036
The Need in Idiopathic Pulmonary Fibrosis (IPF)
IPF is a rare chronic progressive pulmonary disease with abnormal scarring of the lung blocking the movement of oxygen into the bloodstream.
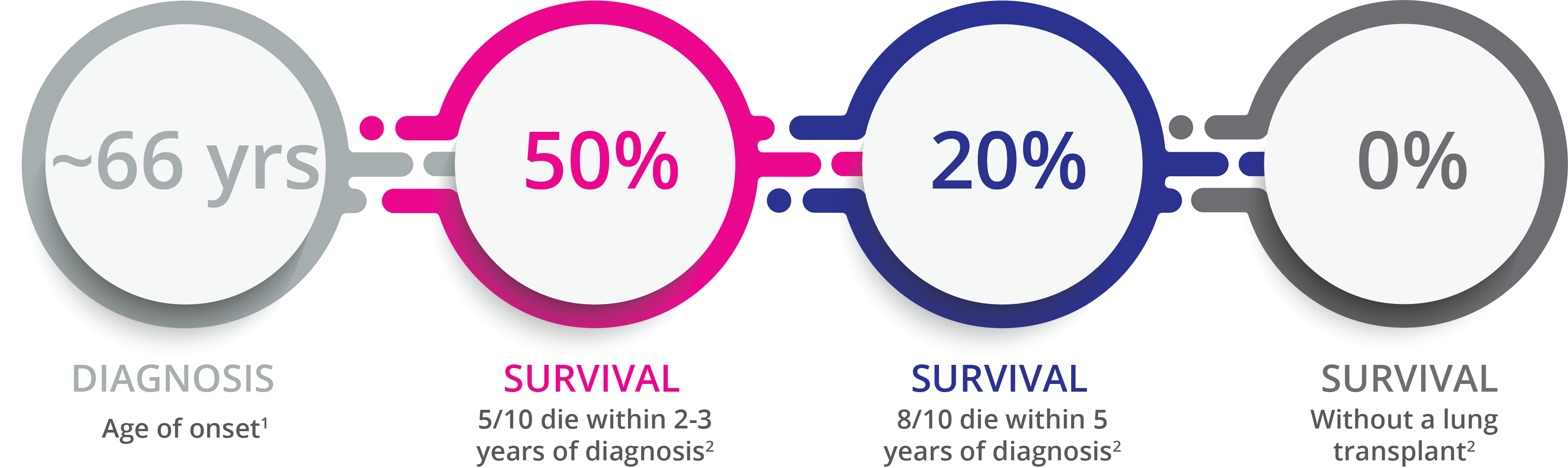
- Castriotta, R. J. (2010). Workshop on Idiopathic Pulmonary Fibrosis in Older Adults. Chest, 138(3), 693–703;
- Sharif, R. (2017). Overview of Idiopathic Pulmonary Fibrosis (IPF) and Evidence-Based Guidelines. Am J Manag Care, 23(11), 176–182
